Why do we use zinc in our primers?
The first question to answer is what is a primer for? Here are some answers:
- Substrate adhesion – maximising adhesion between metal and the protective paint.
- Increasing durability of the coating system.
- Preventing contamination on the metal staining the topcoat.
- Sacrificing itself to provide galvanic protection.
So, primer has lots of benefits, and zinc primers are used when protecting metal to maximise on these benefits.
What’s galvanic protection?
Chemistry tells us that if two dissimilar metals are coupled together and in conductive contact, the more reactive metal will form the anode in the corrosion cell. This will in turn dissolve to protect the more noble cathode.
As an example, when steel is coated with zinc in a clean metallic pathway (see below) the zinc will dissolve, protecting the steel to provide cathodic protection. The zinc-anode protects the steel-cathode. The zinc is therefore known as a sacrificial anode.
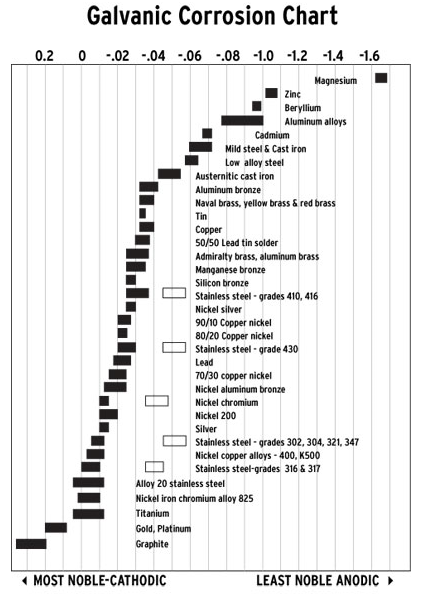
Below is a table showing metal’s nobility; note that zinc is less noble than steel, which is why it works well as a sacrificial anode.
What to look for in zinc primers
Although zinc phosphate primers are readily available, they won’t provide cathodic protection as they typically contain only around 27% zinc dust. At this level they offer corrosion resistance but a minimum of around 77% zinc is required to be called a zinc rich coating offering cathodic protection. In contrast, Rustbuster Zinc Rich Epoxy Anode contains 93% zinc dust and Rustbuster Cold Galvanising’s 90%.
What’s a clean metallic pathway?
For cathodic protection to work properly it’s important that the substrate to be coated is cleaned thoroughly. Rustbuster have written a blog on Rust Removal Options that talks about surface cleaning standards. Mechanical rust removal of some sort (e.g. blast cleaning, MBX bristle blasting) will need to be done to get the benefits of a zinc rich system as metal-to-metal contact is essential.
Summary: using zinc rich primers
In conclusion:
- When using a zinc primer on your vehicle, the zinc is the sacrificial anode that protects the metal of the vehicle’s chassis, body or other components (i.e. the noble cathode).
- To be classed as zinc rich, a primer needs a minimum of 77% zinc dust.
- Any zinc phosphate primer containing less than 27% zinc dust will provide less corrosion resistance to the substrate it’s protecting.
- When using all zinc primers, preparation is always key. Bad preparation when using any paint will result in coating failure.




